In free radical or addition polymerization, an initiator is used to break what type of bonds?
Gratuitous-radical polymerization (FRP) is a method of polymerization, by which a polymer forms by the successive improver of free-radical edifice blocks. Free radicals can exist formed by a number of different mechanisms, commonly involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.
Free-radical polymerization is a fundamental synthesis route for obtaining a wide variety of different polymers and materials composites. The relatively non-specific nature of complimentary-radical chemical interactions makes this ane of the nearly versatile forms of polymerization available and allows facile reactions of polymeric free-radical chain ends and other chemicals or substrates. In 2001, 40 billion of the 110 billion pounds of polymers produced in the Usa were produced by free-radical polymerization.[1]
Gratuitous-radical polymerization is a type of chain-growth polymerization, along with anionic, cationic and coordination polymerization.
A chain polymerization in which the kinetic-chain carriers are radicals.
Note: Usually, the growing chain end bears an unpaired electron.[2]
Initiation [edit]
Initiation is the first step of the polymerization process. During initiation, an active center is created from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators. Radical initiation works best on the carbon–carbon double bond of vinyl monomers and the carbon–oxygen double bond in aldehydes and ketones.[one] Initiation has two steps. In the first pace, one or 2 radicals are created from the initiating molecules. In the 2d footstep, radicals are transferred from the initiator molecules to the monomer units present. Several choices are available for these initiators.
Types of initiation and the initiators [edit]
- Thermal decomposition
- The initiator is heated until a bond is homolytically cleaved, producing two radicals (Figure 1). This method is used most oftentimes with organic peroxides or azo compounds.[3]
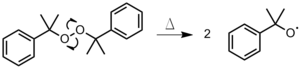
Figure ane: Thermal decomposition of dicumyl peroxide
- Photolysis
- Radiation cleaves a bail homolytically, producing two radicals (Effigy 2). This method is used nigh often with metallic iodides, metal alkyls, and azo compounds.[3] Photoinitiation can besides occur past bi-molecular H abstraction when the radical is in its lowest triplet excited state.[4] An acceptable photoinitiator system should fulfill the post-obit requirements:[4]
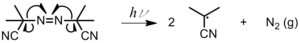
Figure two: Photolysis of azoisobutylnitrile (AIBN)
-
- High absorptivity in the 300–400 nm range.
- Efficient generation of radicals capable of attacking the alkene double bond of vinyl monomers.
- Acceptable solubility in the binder system (prepolymer + monomer).
- Should non impart yellowing or unpleasant odors to the cured material.
- The photoinitiator and any byproducts resulting from its utilize should be non-toxic.
- Redox reactions
- Reduction of hydrogen peroxide or an alkyl hydrogen peroxide by atomic number 26 (Figure 3).[3] Other reductants such equally Crtwo+, V2+, Tiiii+, Coii+, and Cu+ tin can be employed in place of ferrous ion in many instances.[one]
Figure 3: Redox reaction of hydrogen peroxide and iron.
- Persulfates
- The dissociation of a persulfate in the aqueous phase (Figure 4). This method is useful in emulsion polymerizations, in which the radical diffuses into a hydrophobic monomer-containing droplet.[three]
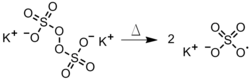
Figure iv: Thermal degradation of a persulfate
- Ionizing radiation
- α-, β-, γ-, or x-rays cause ejection of an electron from the initiating species, followed by dissociation and electron capture to produce a radical (Figure 5).[3]

Effigy 5: The three steps involved in ionizing radiations: ejection, dissociation, and electron-capture
- Electrochemical
- Electrolysis of a solution containing both monomer and electrolyte. A monomer molecule will receive an electron at the cathode to become a radical anion, and a monomer molecule will give up an electron at the anode to form a radical cation (Figure 6). The radical ions then initiate free radical (and/or ionic) polymerization. This blazon of initiation is especially useful for coating metal surfaces with polymer films.[5]
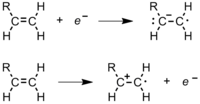
Figure 6: (Elevation) Germination of radical anion at the cathode; (bottom) germination of radical cation at the anode
- Plasma
- A gaseous monomer is placed in an electric discharge at low pressures under weather where a plasma (ionized gaseous molecules) is created. In some cases, the system is heated and/or placed in a radiofrequency field to assist in creating the plasma.[one]
- Sonication
- Loftier-intensity ultrasound at frequencies beyond the range of human hearing (xvi kHz) can be applied to a monomer. Initiation results from the effects of cavitation (the formation and collapse of cavities in the liquid). The collapse of the cavities generates very high local temperatures and pressures. This results in the formation of excited electronic states, which in turn pb to bond breakage and radical germination.[one]
- Ternary initiators
- A ternary initiator is the combination of several types of initiators into ane initiating arrangement. The types of initiators are called based on the backdrop they are known to induce in the polymers they produce. For case, poly(methyl methacrylate) has been synthesized by the ternary system benzoyl peroxide-3,half dozen-bis(o-carboxybenzoyl)-N-isopropylcarbazole-di-η5-indenylzicronium dichloride (Figure 7).[vi] [7] This type of initiating system contains a metallocene, an initiator, and a heteroaromatic diketo carboxylic acid. Metallocenes in combination with initiators advance polymerization of poly(methyl methacrylate) and produce a polymer with a narrower molecular weight distribution. The example shown here consists of indenylzirconium (a metallocene) and benzoyl peroxide (an initiator). Also, initiating systems containing heteroaromatic diketo carboxylic acids, such as iii,vi-bis(o-carboxybenzoyl)-Due north-isopropylcarbazole in this instance, are known to catalyze the decomposition of benzoyl peroxide. Initiating systems with this particular heteroaromatic diket carboxylic acid are besides known to have effects on the microstructure of the polymer. The combination of all of these components—a metallocene, an initiator, and a heteroaromatic diketo carboxylic acid—yields a ternary initiating system that was shown to advance the polymerization and produce polymers with enhanced heat resistance and regular microstructure.[6] [7]

Figure 7: benzoyl peroxide-3,six-bis(o-carboxybenzoyl)-Northward-isopropylcarbazole-di-ηv-indenylzicronium dichloride
Initiator efficiency [edit]
Due to side reactions and inefficient synthesis of the radical species, chain initiation is not 100% [ analyze ]. The efficiency cistron f is used to describe the effective radical concentration. The maximal value of f is ane, only typical values range from 0.3 to 0.8. The post-obit is a list of reactions that subtract the efficiency of the initiator.
- Primary recombination
- Two radicals recombine before initiating a chain (Figure eight). This occurs within the solvent muzzle, meaning that no solvent has yet come between the new radicals.[3]
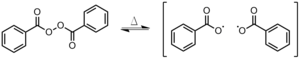
Effigy eight: Primary recombination of BPO; brackets indicate that the reaction is happening within the solvent cage
- Other recombination pathways
- Ii radical initiators recombine before initiating a chain, but not in the solvent cage (Effigy 9).[3]
![]()
Figure nine: Recombination of phenyl radicals from the initiation of BPO outside the solvent muzzle
- Side reactions
- One radical is produced instead of the three radicals that could exist produced (Figure ten).[iii]
Effigy 10: Reaction of polymer concatenation R with other species in reaction
Propagation [edit]
During polymerization, a polymer spends most of its time in increasing its chain length, or propagating. After the radical initiator is formed, it attacks a monomer (Figure 11).[8] In an ethene monomer, 1 electron pair is held securely betwixt the two carbons in a sigma bond. The other is more loosely held in a pi bond. The complimentary radical uses one electron from the pi bond to course a more stable bond with the carbon atom. The other electron returns to the second carbon cantlet, turning the whole molecule into another radical. This begins the polymer concatenation. Figure 12 shows how the orbitals of an ethylene monomer interact with a radical initiator.[9]

Figure 11: Phenyl initiator from benzoyl peroxide (BPO) attacks a styrene molecule to start the polymer concatenation.
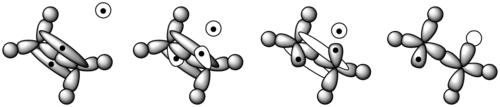
Effigy 12: An orbital drawing of the initiator attack on ethylene molecule, producing the showtime of the polyethylene chain.
Once a concatenation has been initiated, the chain propagates (Effigy 13) until there are no more than monomers (living polymerization) or until termination occurs. There may exist anywhere from a few to thousands of propagation steps depending on several factors such equally radical and concatenation reactivity, the solvent, and temperature.[x] [11] The mechanism of chain propagation is every bit follows:
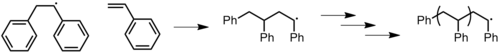
Figure 13: Propagation of polystyrene with a phenyl radical initiator.
Termination [edit]
Chain termination is inevitable in radical polymerization due to the loftier reactivity of radicals. Termination can occur by several different mechanisms. If longer chains are desired, the initiator concentration should be kept depression; otherwise, many shorter chains will issue.[iii]
- Combination of two agile concatenation ends: 1 or both of the following processes may occur.
- Combination: 2 chain ends simply couple together to course ane long chain (Figure 14). 1 tin determine if this mode of termination is occurring by monitoring the molecular weight of the propagating species: combination will upshot in doubling of molecular weight. Besides, combination will effect in a polymer that is C2 symmetric almost the bespeak of the combination.[9]
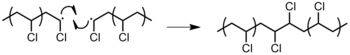
Figure 14: Termination past the combination of 2 poly(vinyl chloride) (PVC) polymers.
- Radical disproportionation: a hydrogen atom from one chain cease is bathetic to another, producing a polymer with a terminal unsaturated grouping and a polymer with a terminal saturated grouping (Figure fifteen).[five]

Figure 15: Termination by disproportionation of poly(methyl methacrylate).
- Combination: 2 chain ends simply couple together to course ane long chain (Figure 14). 1 tin determine if this mode of termination is occurring by monitoring the molecular weight of the propagating species: combination will upshot in doubling of molecular weight. Besides, combination will effect in a polymer that is C2 symmetric almost the bespeak of the combination.[9]
- Combination of an active chain stop with an initiator radical (Effigy 16).[3]
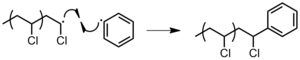
Figure 16: Termination of PVC by reaction with radical initiator.
- Interaction with impurities or inhibitors. Oxygen is the common inhibitor. The growing chain volition react with molecular oxygen, producing an oxygen radical, which is much less reactive (Effigy 17). This significantly slows down the rate of propagation. Nitrobenzene, butylated hydroxyl toluene, and diphenyl picryl hydrazyl (DPPH, Figure eighteen) are a few other inhibitors. The latter is an especially effective inhibitor because of the resonance stabilization of the radical.[iii]

Effigy 17: Inhibition of polystyrene propagation due to reaction of polymer with molecular oxygen.
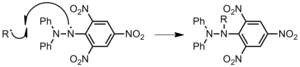
Figure 18: Inhibition of polymer chain, R, past DPPH.
Concatenation transfer [edit]
Contrary to the other modes of termination, chain transfer results in the destruction of only one radical, but as well the creation of another radical. Often, however, this newly created radical is not capable of further propagation. Like to disproportionation, all chain-transfer mechanisms also involve the abstraction of a hydrogen or other atom. There are several types of chain-transfer mechanisms.[iii]
- To solvent: a hydrogen atom is bathetic from a solvent molecule, resulting in the formation of radical on the solvent molecules, which volition not propagate further (Effigy 19). The effectiveness of chain transfer involving solvent molecules depends on the amount of solvent present (more solvent leads to greater probability of transfer), the strength of the bond involved in the abstraction step (weaker bail leads to greater probability of transfer), and the stability of the solvent radical that is formed (greater stability leads to greater probability of transfer). Halogens, except fluorine, are easily transferred.[3]

Figure 19: Chain transfer from polystyrene to solvent.
- To monomer: a hydrogen atom is bathetic from a monomer. While this does create a radical on the affected monomer, resonance stabilization of this radical discourages further propagation (Effigy 20).[3]

Effigy 20: Chain transfer from polypropylene to monomer.
- To initiator: a polymer concatenation reacts with an initiator, which terminates that polymer chain, but creates a new radical initiator (Figure 21). This initiator can and so begin new polymer chains. Therefore, opposite to the other forms of concatenation transfer, concatenation transfer to the initiator does permit for further propagation. Peroxide initiators are especially sensitive to concatenation transfer.[iii]

- To polymer: the radical of a polymer chain abstracts a hydrogen atom from somewhere on another polymer concatenation (Figure 22). This terminates the growth of ane polymer chain, but allows the other to co-operative and resume growing. This reaction step changes neither the number of polymer chains nor the number of monomers which have been polymerized, so that the number-average caste of polymerization is unaffected.[12]

Figure 22: Concatenation transfer from polypropylene to courage of some other polypropylene.
Furnishings of chain transfer: The virtually obvious consequence of chain transfer is a decrease in the polymer chain length. If the rate of transfer is much larger than the rate of propagation, then very minor polymers are formed with chain lengths of 2-v repeating units (telomerization).[13] The Mayo equation estimates the influence of chain transfer on chain length (xn ): . Where ktr is the rate abiding for chain transfer and mp is the rate constant for propagation. The Mayo equation assumes that transfer to solvent is the major termination pathway.[3] [xiv]
Methods [edit]
There are four industrial methods of radical polymerization:[3]
- Majority polymerization: reaction mixture contains only initiator and monomer, no solvent.
- Solution polymerization: reaction mixture contains solvent, initiator, and monomer.
- Suspension polymerization: reaction mixture contains an aqueous phase, water-insoluble monomer, and initiator soluble in the monomer aerosol (both the monomer and the initiator are hydrophobic).
- Emulsion polymerization: similar to suspension polymerization except that the initiator is soluble in the aqueous stage rather than in the monomer droplets (the monomer is hydrophobic, and the initiator is hydrophilic). An emulsifying agent is besides needed.
Other methods of radical polymerization include the following:
- Template polymerization: In this process, polymer chains are allowed to grow along template macromolecules for the greater function of their lifetime. A well-called template can touch the rate of polymerization besides as the molar mass and microstructure of the girl polymer. The molar mass of a daughter polymer tin be upwardly to 70 times greater than those of polymers produced in the absence of the template and can be higher in tooth mass than the templates themselves. This is considering of retardation of the termination for template-associated radicals and past hopping of a radical to the neighboring template after reaching the cease of a template polymer.[15]
- Plasma polymerization: The polymerization is initiated with plasma. A variety of organic molecules including alkenes, alkynes, and alkanes undergo polymerization to high molecular weight products under these conditions. The propagation mechanisms appear to involve both ionic and radical species. Plasma polymerization offers a potentially unique method of forming thin polymer films for uses such as thin-film capacitors, antireflection coatings, and various types of thin membranes.[one]
- Sonication: The polymerization is initiated by high-intensity ultrasound. Polymerization to high molecular weight polymer is observed but the conversions are depression (<15%). The polymerization is cocky-limiting considering of the loftier viscosity produced even at low conversion. High viscosity hinders cavitation and radical production.[1]
Reversible deactivation radical polymerization [edit]
Also known equally living radical polymerization, controlled radical polymerization, reversible deactivation radical polymerization (RDRP) relies on completely pure reactions, preventing termination caused by impurities. Because these polymerizations finish only when in that location is no more monomer, polymerization tin continue upon the addition of more monomer. Cake copolymers can be made this manner. RDRP allows for control of molecular weight and dispersity. However, this is very difficult to reach and instead a pseudo-living polymerization occurs with only partial command of molecular weight and dispersity.[15] ATRP and RAFT are the main types of complete radical polymerization.
- Atom transfer radical polymerization (ATRP): based on the formation of a carbon-carbon bail by atom transfer radical improver. This method, independently discovered in 1995 past Mitsuo Sawamoto[16] and by Jin-Shan Wang and Krzysztof Matyjaszewski,[17] [eighteen] requires reversible activation of a dormant species (such every bit an alkyl halide) and a transition element halide goad (to actuate dormant species).[3]
- Reversible Improver-Fragmentation Concatenation-Transfer Polymerization (RAFT): requires a compound that can act as a reversible concatenation-transfer agent, such every bit dithio compound.[three]
- Stable Free Radical Polymerization (SFRP): used to synthesize linear or branched polymers with narrow molecular weight distributions and reactive end groups on each polymer concatenation. The process has too been used to create block co-polymers with unique backdrop. Conversion rates are near 100% using this process only require temperatures of virtually 135 °C. This process is most commonly used with acrylates, styrenes, and dienes. The reaction scheme in Figure 23 illustrates the SFRP procedure.[19]

Figure 23: Reaction scheme for SFRP.
Because the concatenation stop is functionalized with the TEMPO molecule (Figure 24), premature termination by coupling is reduced. Every bit with all living polymerizations, the polymer chain grows until all of the monomer is consumed.[19]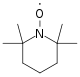
Figure 24: TEMPO molecule used to functionalize the chain ends.
Kinetics [edit]
In typical concatenation growth polymerizations, the reaction rates for initiation, propagation and termination tin be described as follows:
where f is the efficiency of the initiator and md, kp, and kt are the constants for initiator dissociation, chain propagation and termination, respectively. [I] [Thousand] and [K•] are the concentrations of the initiator, monomer and the active growing chain.
Under the steady-state approximation, the concentration of the active growing bondage remains abiding, i.due east. the rates of initiation and of termination are equal. The concentration of active chain can be derived and expressed in terms of the other known species in the system.
In this case, the rate of concatenation propagation can be further described using a function of the initiator and monomer concentrations[20] [21]
The kinetic chain length v is a measure of the boilerplate number of monomer units reacting with an active center during its lifetime and is related to the molecular weight through the mechanism of the termination. Without chain transfer, the kinetic chain length is only a role of propagation charge per unit and initiation rate.[22]
Assuming no chain-transfer event occurs in the reaction, the number average degree of polymerization Pn can be correlated with the kinetic chain length. In the example of termination past disproportionation, i polymer molecule is produced per every kinetic chain:
Termination by combination leads to one polymer molecule per two kinetic chains:[twenty]
Any mixture of both these mechanisms tin be described by using the value δ , the contribution of disproportionation to the overall termination process:
If chain transfer is considered, the kinetic chain length is not affected by the transfer procedure considering the growing free-radical center generated by the initiation footstep stays alive subsequently whatever chain-transfer event, although multiple polymer bondage are produced. However, the number average degree of polymerization decreases as the chain transfers, since the growing chains are terminated by the concatenation-transfer events. Taking into business relationship the chain-transfer reaction towards solvent S, initiator I, polymer P, and added chain-transfer agent T. The equation of Pn will exist modified as follows:[23]
Information technology is usual to define chain-transfer constants C for the dissimilar molecules
- , , , ,
Thermodynamics [edit]
In chain growth polymerization, the position of the equilibrium between polymer and monomers can exist determined by the thermodynamics of the polymerization. The Gibbs free free energy (ΔGp) of the polymerization is commonly used to quantify the tendency of a polymeric reaction. The polymerization will be favored if ΔGp < 0; if ΔGp > 0, the polymer will undergo depolymerization. According to the thermodynamic equation ΔG = ΔH – TΔS, a negative enthalpy and an increasing entropy will shift the equilibrium towards polymerization.
In full general, the polymerization is an exothermic process, i.east. negative enthalpy change, since addition of a monomer to the growing polymer chain involves the conversion of π bonds into σ bonds, or a ring–opening reaction that releases the ring tension in a cyclic monomer. Meanwhile, during polymerization, a large amount of small molecules are associated, losing rotation and translational degrees of freedom. As a result, the entropy decreases in the system, ΔSp < 0 for nearly all polymerization processes. Since depolymerization is nearly e'er entropically favored, the ΔHp must then exist sufficiently negative to recoup for the unfavorable entropic term. Only then volition polymerization be thermodynamically favored by the resulting negative ΔGp.
In practice, polymerization is favored at low temperatures: TΔSp is small. Depolymerization is favored at high temperatures: TΔSp is large. As the temperature increases, ΔGp go less negative. At a certain temperature, the polymerization reaches equilibrium (rate of polymerization = rate of depolymerization). This temperature is called the ceiling temperature (Tc). ΔGp = 0.[24]
Stereochemistry [edit]
The stereochemistry of polymerization is concerned with the divergence in atom connectivity and spatial orientation in polymers that has the aforementioned chemical composition.
Hermann Staudinger studied the stereoisomerism in chain polymerization of vinyl monomers in the late 1920s, and information technology took another two decades for people to fully appreciate the idea that each of the propagation steps in the polymer growth could give rise to stereoisomerism. The major milestone in the stereochemistry was established by Ziegler and Natta and their coworkers in 1950s, every bit they developed metal based catalyst to synthesize stereoregular polymers. The reason why the stereochemistry of the polymer is of particular interest is because the physical beliefs of a polymer depends not only on the full general chemical limerick just also on the more subtle differences in microstructure.[25] Atactic polymers consist of a random arrangement of stereochemistry and are baggy (noncrystalline), soft materials with lower physical strength. The respective isotactic (similar substituents all on the same side) and syndiotactic (like substituents of alternate repeating units on the same side) polymers are usually obtained as highly crystalline materials. Information technology is easier for the stereoregular polymers to pack into a crystal lattice since they are more ordered and the resulting crystallinity leads to higher physical strength and increased solvent and chemical resistance as well every bit differences in other properties that depend on crystallinity. The prime example of the industrial utility of stereoregular polymers is polypropene. Isotactic polypropene is a high-melting (165 °C), strong, crystalline polymer, which is used equally both a plastic and fiber. Atactic polypropene is an amorphous material with an oily to waxy soft appearance that finds use in asphalt blends and formulations for lubricants, sealants, and adhesives, simply the volumes are minuscule compared to that of isotactic polypropene.
When a monomer adds to a radical concatenation finish, there are two factors to consider regarding its stereochemistry: 1) the interaction betwixt the terminal concatenation carbon and the budgeted monomer molecule and 2) the configuration of the penultimate repeating unit in the polymer chain.[5] The final carbon atom has sp2 hybridization and is planar. Consider the polymerization of the monomer CH2=CXY. There are two ways that a monomer molecule can arroyo the terminal carbon: the mirror approach (with like substituents on the same side) or the non-mirror approach (like substituents on opposite sides). If free rotation does not occur before the next monomer adds, the mirror approach will always lead to an isotactic polymer and the not-mirror approach will always lead to a syndiotactic polymer (Figure 25).[five]
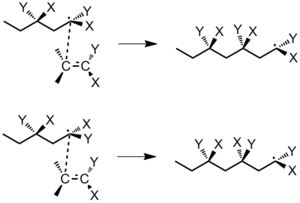
Figure 25: (Pinnacle) germination of isotactic polymer; (bottom) germination of syndiotactic polymer.
Even so, if interactions between the substituents of the penultimate repeating unit and the concluding carbon cantlet are meaning, then conformational factors could cause the monomer to add to the polymer in a manner that minimizes steric or electrostatic interaction (Effigy 26).[5]
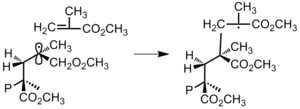
Figure 26: Penultimate unit interactions cause monomer to add in a mode that minimizes steric hindrance between substituent groups. (P represents polymer concatenation.)
Reactivity [edit]
Traditionally, the reactivity of monomers and radicals are assessed past the means of copolymerization data. The Q–due east scheme, the near widely used tool for the semi-quantitative prediction of monomer reactivity ratios, was first proposed by Alfrey and Toll in 1947.[26] The scheme takes into account the intrinsic thermodynamic stability and polar furnishings in the transition state. A given radical and a monomer are considered to have intrinsic reactivities Pi and Qj, respectively.[27] The polar furnishings in the transition land, the supposed permanent electric charge carried by that entity (radical or molecule), is quantified by the factor e, which is a constant for a given monomer, and has the same value for the radical derived from that specific monomer. For addition of monomer 2 to a growing polymer chain whose agile finish is the radical of monomer 1, the charge per unit abiding, k 12, is postulated to be related to the iv relevant reactivity parameters by
The monomer reactivity ratio for the addition of monomers 1 and 2 to this chain is given by[27] [28]
For the copolymerization of a given pair of monomers, the two experimental reactivity ratios rane and r2 let the evaluation of (Qi/Q2) and (eone – east2). Values for each monomer can then be assigned relative to a reference monomer, unremarkably called as styrene with the arbitrary values Q = 1.0 and eastward = –0.viii.[28]
Applications [edit]
Free radical polymerization has found applications including the industry of polystyrene, thermoplastic cake copolymer elastomers,[29] cardiovascular stents,[30] chemical surfactants[31] and lubricants. Block copolymers are used for a broad multifariousness of applications including adhesives, footwear and toys.
Free radical polymerization has uses in research likewise, such every bit in the functionalization of carbon nanotubes.[32] CNTs intrinsic electronic backdrop lead them to form large aggregates in solution, precluding useful applications. Adding small chemic groups to the walls of CNT can eliminate this propensity and tune the response to the surrounding environs. The use of polymers instead of smaller molecules can alter CNT properties (and conversely, nanotubes can modify polymer mechanical and electronic properties).[29] For instance, researchers coated carbon nanotubes with polystyrene by first polymerizing polystyrene via concatenation radical polymerization and later on mixing information technology at 130 °C with carbon nanotubes to generate radicals and graft them onto the walls of carbon nanotubes (Effigy 27).[33] Chain growth polymerization ("grafting to") synthesizes a polymer with predetermined backdrop. Purification of the polymer can be used to obtain a more uniform length distribution before grafting. Conversely, "grafting from", with radical polymerization techniques such as atom transfer radical polymerization (ATRP) or nitroxide-mediated polymerization (NMP), allows rapid growth of high molecular weight polymers.
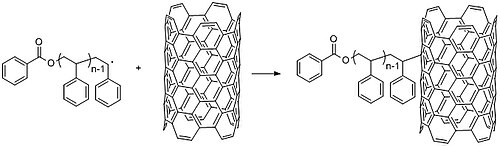
Figure 27: Grafting of a polystyrene gratis radical onto a single-walled carbon nanotube.
Radical polymerization also aids synthesis of nanocomposite hydrogels.[34] These gels are made of water-swellable nano-scale clay (especially those classed as smectites) enveloped by a network polymer. They are frequently biocompatible and accept mechanical properties (such as flexibility and strength) that promise applications such equally synthetic tissue. Synthesis involves costless radical polymerization. The general synthesis procedure is depicted in Figure 28. Clay is dispersed in water, where it forms very minor, porous plates. Next the initiator and a catalyst are added, followed by calculation the organic monomer, generally an acrylamide or acrylamide derivative. The initiator is chosen to have stronger interaction with the clay than the organic monomer, so information technology preferentially adsorbs to the dirt surface. The mixture and h2o solvent is heated to initiate polymerization. Polymers grow off the initiators that are in turn jump to the dirt. Due to recombination and disproportionation reactions, growing polymer chains bind to one some other, forming a potent, cantankerous-linked network polymer, with clay particles interim equally branching points for multiple polymer chain segments.[35] Free radical polymerization used in this context allows the synthesis of polymers from a wide variety of substrates (the chemistries of suitable clays vary). Termination reactions unique to concatenation growth polymerization produce a material with flexibility, mechanical forcefulness and biocompatibility.

Figure 28: General synthesis procedure for a nanocomposite hydrogel.
Electronics [edit]
The radical polymer drinking glass PTMA is about ten times more electrically conductive than mutual semiconducting polymers. PTMA is in a class of electrically agile polymers that could find use in transparent solar cells, antistatic and antiglare coatings for mobile telephone displays, antistatic coverings for aircraft to protect confronting lightning strikes, flexible wink drives, and thermoelectric devices, which convert electricity into heat and the opposite. Widespread applied applications require increasing conductivity another 100 to one,000 times.
The polymer was created using deprotection, which involves replacing a specific hydrogen atom in the pendant group with an oxygen atom. The resulting oxygen cantlet in PTMA has one unpaired electron in its outer shell, making it acquiescent to transporting charge. The deprotection pace tin can lead to iv singled-out chemic functionalities, two of which are promising for increasing conductivity.[36]
Encounter also [edit]
- Anionic improver polymerization
- Chain-growth polymerisation
- Chain transfer
- Cobalt-mediated radical polymerization
- Living polymerization
- Nitroxide mediated radical polymerization
- Polymer
- Polymerization
- Reversible-deactivation radical polymerization
- Step-growth polymerization
References [edit]
- ^ a b c d e f g Odian, George (2004). Principles of Polymerization (4th ed.). New York: Wiley-Interscience. ISBN978-0-471-27400-1.
- ^ Jenkins, A. D.; Kratochvíl, P.; Stepto, R. F. T.; Suter, U. W. (1996). "Glossary of basic terms in polymer science (IUPAC Recommendations 1996)". Pure and Practical Chemical science. 68 (12): 2287–2311. doi:10.1351/pac199668122287.
- ^ a b c d e f k h i j k 50 one thousand north o p q r southward Cowie, J. K. 1000.; Arrighi, Valeria (2008). Polymers: Chemical science and Physics of Modern Materials (3rd ed.). Scotland: CRC Press. ISBN978-0-8493-9813-one.
- ^ a b Hageman, H. J. (1985). "Photoinitiators for Gratis Radical Polymerization". Progress in Organic Coatings. 13 (2): 123–150. doi:10.1016/0033-0655(85)80021-2.
- ^ a b c d e Stevens, Malcolm P. (1999). Polymer Chemistry: An Introduction. New York: Oxford Academy Press. ISBN978-0-19-512444-6.
- ^ a b Islamova, R. K.; Puzin, Y. I.; Kraikin, V. A.; Fatykhov, A. A.; Dzhemilev, U. One thousand. (2006). "Decision-making the Polymerization of Methyl Methacrylate with Ternary Initiating Systems". Russian Journal of Practical Chemistry. 79 (ix): 1509–1513. doi:10.1134/S1070427206090229.
- ^ a b Islamova, R. One thousand.; Puzin, Y. I.; Fatykhov, A. A.; Monakov, Y. B. (2006). "A Ternary Initiating Organisation for Costless Radical Polymerization of Methyl Methacrylate". Polymer Science, Serial B. 48 (three): 130–133. doi:10.1134/S156009040605006X.
- ^ "Improver Polymerization". Materials Earth Modules. June 2009. Retrieved 1 April 2010.
- ^ a b "Polymer Synthesis". Case Western Reserve University. 2009. Archived from the original on vii Feb 2010. Retrieved 10 March 2010.
- ^ Leach, Marking R. "Radical Chemistry". Chemogenesis. Retrieved 2 April 2010.
- ^ Pojman, John A.; Jason Willis; Dionne Fortenberry; Victor Ilyashenko; Akhtar Thou. Khan (1995). "Factors affecting propagating fronts of add-on polymerization: Velocity, front end curvature, temperature contour, conversion, and molecular weight distribution". Journal of Polymer Science Part A: Polymer Chemistry. 33 (4): 643–652. Bibcode:1995JPoSA..33..643P. doi:10.1002/pola.1995.080330406.
- ^ Rudin, Alfred The Elements of Polymer Science and Engineering (Academic Press 1982) p.220 ISBN 0-12-601680-i
- ^ Rudin, Alfred The Elements of Polymer Science and Engineering science (Academic Printing 1982) p.212 ISBN 0-12-601680-one
- ^ The Mayo equation for chain transfer should not be confused with the Mayo–Lewis equation for copolymers.
- ^ a b Colombani, Daniel (1997). "Chain-Growth Command in Free Radical Polymerization". Progress in Polymer Science. 22 (8): 1649–1720. doi:10.1016/S0079-6700(97)00022-i.
- ^ Kato, M; Kamigaito, M; Sawamoto, K; Higashimura, T (1995). "Polymerization of Methyl Methacrylate with the Carbon Tetrachloride / Dichlorotris-(triphenylphosphine)ruthenium(II) / Methylaluminum Bis(2,six-di-tert-butylphenoxide) Initiating Arrangement: Possibility of Living Radical Polymerization". Macromolecules. 28 (v): 1721–1723. Bibcode:1995MaMol..28.1721K. doi:10.1021/ma00109a056.
- ^ Wang, J-S; Matyjaszewski, K (1995). "Controlled/"living" radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes". J. Am. Chem. Soc. 117 (twenty): 5614–5615. doi:x.1021/ja00125a035.
- ^ "The 2011 Wolf Prize in Chemistry". Wolf Fund. Archived from the original on 17 May 2007. Retrieved 21 February 2011.
- ^ a b "Stable Free Radical Polymerization". Xerox Corp. 2010. Archived from the original on 28 November 2003. Retrieved 10 March 2010.
- ^ a b Cowie, J. M. K. (1991). Polymers: Chemistry and Physics of Mod Materials (2nd ed.). Blackie (Us: Chapman & Hall). pp. 58–lx. ISBN978-0-216-92980-vii.
- ^ Rudin, Alfred The Elements of Polymer Science and Engineering (Academic Press 1982) pp.195-9 ISBN 0-12-601680-1
- ^ Rudin, Alfred The Elements of Polymer Scientific discipline and Engineering science (Academic Press 1982) p.209 ISBN 0-12-601680-1
- ^ Rudin, Alfred The Elements of Polymer Science and Engineering (Academic Press 1982) p.214 ISBN 0-12-601680-1
- ^ Fried, Joel R. Polymer Scientific discipline & Applied science (2d ed., Prentice-Hall 2003) p.39 ISBN 0-xiii-018168-4
- ^ Clark, Jim (2003). "The Polymerization of Alkenes". ChemGuide. Retrieved 1 April 2010.
- ^ Alfrey, Turner; Toll, Charles C. (1947). "Relative reactivities in vinyl copolymerization". Periodical of Polymer Science. 2 (1): 101–106. Bibcode:1947JPoSc...2..101A. doi:10.1002/politician.1947.120020112.
- ^ a b Allcock H.R., Lampe F.W. and Marker J.E. Gimmicky Polymer Chemistry (third ed., Pearson Prentice-Hall 2003) p.364 ISBN 0-thirteen-065056-0
- ^ a b Rudin, Alfred The Elements of Polymer Science and Engineering science (Academic Press 1982) p.289 ISBN 0-12-601680-i
- ^ a b Braunecker, Westward. A.; Yard. Matyjaszewski (2007). "Controlled/living radical polymerization: Features, developments, and perspectives". Progress in Polymer Science. 32 (1): 93–146. doi:ten.1016/j.progpolymsci.2006.11.002.
- ^ Richard, R. E.; M. Schwarz; Due south. Ranade; A. K. Chan; K. Matyjaszewski; B. Sumerlin (2005). "Evaluation of acrylate-based block copolymers prepared by cantlet transfer radical polymerization as matrices for paclitaxel delivery from coronary stents". Biomacromolecules. half-dozen (6): 3410–3418. doi:x.1021/bm050464v. PMID 16283773.
- ^ Burguiere, C.; S. Pascual; B. Coutin; A. Polton; One thousand. Tardi; B. Charleux; K. Matyjaszewski; J. P. Vairon (2000). "Amphiphilic block copolymers prepared via controlled radical polymerization equally surfactants for emulsion polymerization". Macromolecular Symposia. 150: 39–44. doi:x.1002/1521-3900(200002)150:ane<39::Assistance-MASY39>three.0.CO;2-D.
- ^ Homenick, C. Thousand.; Yard. Lawson; A. Adronov (2007). "Polymer grafting of carbon nanotubes using living free-radical polymerization". Polymer Reviews. 47 (ii): 265–270. doi:x.1080/15583720701271237.
- ^ Lou, X. D.; C. Detrembleur; Five. Sciannamea; C. Pagnoulle; R. Jerome (2004). "Grafting of alkoxyamine cease-capped (co)polymers onto multi-walled carbon nanotubes". Polymer. 45 (eighteen): 6097–6102. doi:10.1016/j.polymer.2004.06.050.
- ^ Haraguchi, K. (2008). "Nanocomposite hydrogels". Current Stance in Solid Country and Materials Science. 11 (3–4): 47–54. Bibcode:2007COSSM..11...47H. doi:10.1016/j.cossms.2008.05.001.
- ^ Haraguchi, K.; Takehisa T. (2002). "Nanocomposite hydrogels: a unique organic-inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties". Advanced Materials. 14 (16): 1120–1123. doi:10.1002/1521-4095(20020816)xiv:16<1120::AID-ADMA1120>3.0.CO;2-ix.
- ^ Venere, Emil (9 October 2014). "Electrically conductive plastics promising for batteries, solar cells". Purdue University News . Retrieved 13 July 2017.
External links [edit]
- Addition Polymerization
- Free Radical Polymerization (video animation)
- Gratis Radical Polymerization - Chain Transfer
- Costless Radical Vinyl Polymerization
- The Polymerization of Alkenes
- Polymer Synthesis
- Radical Reaction Chemistry
- Stable Free Radical Polymerization
markusparienve1993.blogspot.com
Source: https://en.wikipedia.org/wiki/Radical_polymerization


![{\frac {1}{x_{n}}}=\left({\frac {1}{x_{n}}}\right)_{o}+{\frac {k_{{tr}}[solvent]}{k_{p}[monomer]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3af10ecbdc45b856a8cd76ecc9502f63fc6bdc03)
![v_{i}={\operatorname {d}[M\cdot ]/\operatorname {d}t}=2k_{d}f[I]](https://wikimedia.org/api/rest_v1/media/math/render/svg/f02573160d99ce28cd15dfe6c9f111e9c47c082d)
![v_{p}=k_{p}[M][M\cdot ]](https://wikimedia.org/api/rest_v1/media/math/render/svg/bc7bb155b647c8e9a58d7d300e71a52898b8f221)
![v_{t}={-\operatorname {d}[M\cdot ]/\operatorname {d}t}=2k_{t}[M\cdot ]^{2}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e451f440b60e6b1e9fbe627b99a0d6eb734aeea4)
![[M\cdot ]=\left({\frac {k_{d}[I]f}{k_{t}}}\right)^{{1/2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/92af6e128be7fa6a566a3590c6f267e601e5e3e2)
![{\displaystyle v_{p}={k_{p}}\left({\frac {fk_{d}}{k_{t}}}\right)^{1/2}[I]^{1/2}[M]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8fab2227059300c21a6173d5108ca221645da4cf)
![{\displaystyle \nu ={\frac {v_{p}}{v_{i}}}={\frac {k_{p}[M][M\cdot ]}{2fk_{d}[I]}}={\frac {k_{p}[M]}{2(fk_{d}k_{t}[I])^{1/2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/60318ebbc0960f885949ea451e3692f7cebae026)



![{\displaystyle {\frac {1}{x_{n}}}={\frac {2k_{t,d}+k_{t,c}}{{k_{p}}^{2}[M]^{2}}}v_{p}+C_{M}+C_{S}{\frac {[S]}{[M]}}+C_{I}{\frac {[I]}{[M]}}+C_{P}{\frac {[P]}{[M]}}+C_{T}{\frac {[T]}{[M]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f87872861546ef6fc87a203d04693d6898ca25d2)









0 Response to "In free radical or addition polymerization, an initiator is used to break what type of bonds?"
Post a Comment